Diamonds are composed of carbon atoms which were topic to extreme strain and warmth. His dissertation requires no scientific background of readers, even whereas investigating the complicated nature, dimension, energy incentives, and attraction between particular atoms – especially the propensity of molecules to grow into more advanced conglomerates and in a predictable fashion.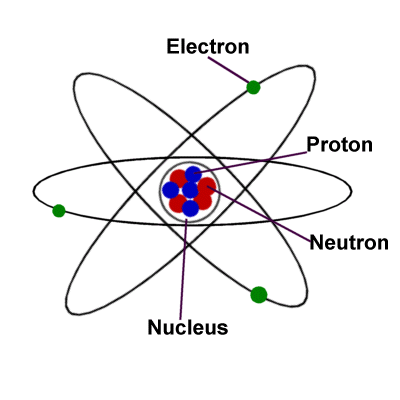
Assuming that hydrogen was the smallest component, the relationship between carbon and hydrogen was used to outline a relative mass unit. Structure of Matter : This very good interactive slideshow from the Nobel Prize web site explains, in 22 slides, all about atoms and the other particles inside them.
This was finished by sending considered one of its electrons right into a “Rydberg state”, through which it no longer exists as a cloud of charge enshrouding the nucleus but instead turns into a ‘wave packet’ that circles the atomic nucleus like a planet around the Solar.
Everyone knows that the Atomic Theory was developed by an English chemist and physicist John Dalton. Atom is the smallest neutral particles in a component. Electrons (or positrons), having mass, could be created from vitality (similar to mass might be transformed to vitality as in the case of the electron-positron annihilation process). Nevertheless, low-energy and low-frequency elementary particles (as described currently in the physicists’ “Commonplace Mannequin”) easily lose their property of consciousness once they grow to be entangled with different particles and decoherence units-in.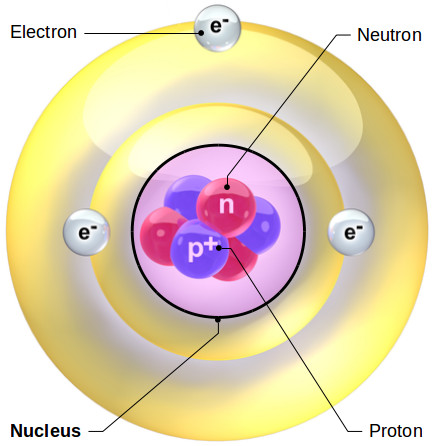
The potential vitality of an electron in an atom is detrimental , its dependence of its place reaches the minimal (essentially the most absolute worth ) contained in the nucleus, and vanishes when the distance from the nucleus goes to infinity , roughly in an inverse proportion to the gap.
I found out that atomic mass is a theoretical mass and not an actual mass. Elements are naturally occurring chemical compounds they usually include atoms of only one variety. A fluorine atom (F) kinds a unfavorable ion (F −) by gaining an electron. Meaning the nucleus of an atom is successfully a giant clump of positive charge.
In September 1803, he found the relative weight of water, carbon dioxide and ammonia and in addition discovered the image representing the component. Hydrogen, helium, carbon, nitrogen are referred to as parts. The equation is now balanced, because the number of atoms of every element are identical on either side of the equation.
Earlier than we focus on the structure of atoms, we have to learn the phrases mass and weight. A new theory that allowed for subatomic particles was wanted. Atoms join collectively to make molecules : for instance, two hydrogen atoms and one oxygen atom mix to make a water molecule. An atom’s electron configuration is the orbital description of the places of the electrons in a typical atom.
Dalton believed that all atoms of the identical ingredient have the same mass. On this relative scale, if oxygen is sixteen instances larger than hydrogen its mass is 16 amu. All matter is made up of assorted combos of those a hundred+ components. Chadwick seen that the atomic number of the elements was decrease than the total atomic mass of the atom.
A specific atom can have the identical number of protons and electrons and most atoms have a minimum of as many neutrons as protons. In response to Dalton, in a sure compound, the atoms of the compound’s parts at all times mix the same way. For further illustration, allow us to contemplate the combustion of natural fuel methane (CH4) in oxygen or air, which yield carbon dioxide (CO2) and water (H2O).
The big majority of an atom’s mass comes from the protons and neutrons that make it up. The overall variety of these particles (referred to as “nucleons”) in a given atom known as the mass quantity It’s a optimistic integer and dimensionless (as a substitute of getting dimension of mass), as a result of it expresses a count.
Critique Of Sir William Bragg’s Ebook
Though early scientists were not capable of measure the mass of individual atoms, they looked for a workable technique that will allow them to outline the mass of an atom. Fermi then noticed that the fission of 1 uranium atom shot off extra neutrons, which then split other atoms, creating chain reactions. Some ancient philosophers believed that matter is infinitely divisible, that any particle, irrespective of how small, can all the time be divided into smaller particles.
A positive hydrogen ion has no electrons, just the one proton and one neutron. Every radioactive element or isotope has what is known as a half-life That is how long it takes half of any sample of atoms of that sort to decay till they grow to be a special steady isotope or aspect.
The Emptiness Of Atomic Space
In 1906 Ernest Rutherford and his assistants, performed the well-known Gold Foil experiment that led to the discoveries of the atomic nucleus and that the atom is usually area.
Molecular Mass of a substance is the sum of the atomic masses of all atoms present in the MF of a substance or molecule. They often involve metals and nonmetals as components lively in ionic bonds are largely from reverse ends of the periodic table with an electronegativity distinction exceeding 1.sixty seven. Being very sturdy, ionic bonds in compounds improve melting points and take a solid type in normal situations.
The periodic table tells you virtually every thing about all of the chemical elements, their atomic numbers, weights, valencies and the categories they belong to. Here’s a variety of articles on the periodic desk of components. So for a long time it was a thriller to scientists how the positively charged protons within the nucleus stayed collectively.
The electromagnetic drive that binds the atoms together to create molecular compounds, come collectively to type an even larger molecule and polymer that makes existence attainable. When Bohr’s atomic structure couldn’t clarify these phenomena, scientists got here up with several ideas together with electrons moving in identical round orbits however in numerous instructions.
Electrons have a relative mass of 0.0005439 (as in contrast with the mass of a neutron being 1) or about 9.109×10-31 kg. Soddy referred to as an atom like this, with a special variety of neutrons, an isotope To get the identify of the isotope we take a look at what number of protons and neutrons it has in its nucleus and add this to the title of the aspect.
Atomic Principle Of Matter
Physics is the department of science pertaining to the examine of various types of matter, their properties, interactions, and transformations, and so forth. When this engaging force within the nucleus is released, the atom is “split” and the superior energy generally known as nuclear power results. Most atoms have three totally different subatomic particles inside them: protons, neutrons, and electrons. A helium atom, with the nucleus proven in pink (and enlarged), embeded in a cloud of electrons.
If an atom has extra or fewer electrons than its atomic quantity, then it turns into respectively negatively or positively charged as a whole; a charged atom is named an ion Electrons have been identified since the late 19th century, principally because of J.J. Thomson ; see historical past of subatomic physics for details.
Balancing Of Chemical Equation
Based on recorded history, the atom concept was developed by the ancient Greeks and the mathematician Democritus. When subjected to external forces, like electrical fields , the shape of an atom might deviate from spherical symmetry The deformation will depend on the sphere magnitude and the orbital type of outer shell electrons, as shown by group-theoretical considerations.
Nitrogen atoms sometimes have 7 neutrons and others have 8 neutrons, and so on. Up to dozens of electrons, that revolve and spin in an space too small to be seen with even probably the most powerful microscopes, create heavy site visitors contained in the atom.
atoms for peace judge jury and executioner, atoms and molecules class 9 ncert solutions, atoms and molecules class 9 ncert
Magnets produce a magnetic field that impacts the orientation of atoms in other nearby matter. The protons and neutrons cluster collectively within the central part of the atom, referred to as the nucleus , and the electrons ‘orbit’ the nucleus. To complicate things a bit extra, we typically discover atoms of a chemical aspect which are a bit completely different to what we anticipate.